Dalton's Law of Partial Pressure
The total pressure of a mixture of carbon dioxide oxygen and helium is 925 kPa. On the basis of experience of 5.

Dalton S Law Of Partial Pressure Ideal Gas Equation Gases And Kinetic Molecular Theory Chemistry Khan Dalton S Law Anatomy And Physiology Ideal Gas Law
The partial pressures of the individual gases in the container.

. Daltons Law of Partial Pressure. It provides the equations plus plenty of examples and practice probl. This chemistry video tutorial explains the concept of daltons law of partial pressure.
In this video I describe daltons law of partial pressure with an animation and also give an question example at the end of the video using daltons law of par. Answer -Oxygen hydrogen and nitrogen gases do not react with each other at 25C so with the help of Daltons law of partial pressure. The partial pressure of the gas is represented by the symbol P with the symbol of the gas in the subscript.
Daltons Law of Partial Pressure 1. P p 1 p 2 p 3. If the partial pressure of carbon dioxide is 273 kPa and the partial.
Daltons Law of Partial Pressures. This Power Point Presentation covers Kinetic Molecular Theory Daltons Law of Partial Pressures Boyles Law Charless Law Gay-Lussacs law the combined gas law and the ideal gas law. The partial pressures of hydrogen oxygen and argon.
In 1801 English chemist John Dalton made observations about steam and air that is published in 1802 and eventually because Daltons. According to Daltons law of partial pressures the total pressure exerted by the mixture of gases is the sum of the partial pressure of every existing. From Daltons law of partial pressure Example 2.
As Dalton understood partial pressures the phenomenon was named after him. For example P o 2 represents partial pressure of oxygen. According to the law of Dalton we can.
Three gases hydrogen oxygen and methane are mixed in a container. What is Daltons Law of. If the water levels within and.
Whereas P is the. Application of Daltons Law. Formulated by John Dalton in the year 1801 Daltons Law or Daltons Law of Partial Pressure states that the total pressure exerted by a mixture of gases is equal to the sum of the partial.
P pa pw 1. P1 P2 P3 are the partial pressures of the various gases in the mixture. Daltons Law of Partial Pressure may be used to calculate the pressure of gases over the surface of a liquid.
The partial pressure is the pressure each gas would exert if it alone occupied the volume of the mixture. Ptotal P1 P2 P3. Daltons Law of partial pressure for moist air can be expressed as.
Whats the partial pressure of carbon dioxide in a container that holds 5 moles of carbon dioxide 3 moles of nitrogen and 1 mole of hydrogen and has a total pressure of 105 atm. Hello dear students this is KULDEEP SIR And this platform is for academics study and foundation batch for jee neet aspirants. How to calculate total pressure and partial pressures from Ideal gas law To convert a into atm L 2 mol 2 multiply by 0986 atmbar.
What is Daltons law of partial pressures An explanation. Daltons Law Core Concepts.

Daltons Law Of Partial Pressures Easy Science Dalton S Law Easy Science Organic Chemistry Study

Dalton S Law Of Partial Pressureslaw Dalton Pressures Partial Dalton S Law Basic Physics Thermodynamics

Dalton S Law Of Partial Pressure Dalton S Law 11th Chemistry Chemistry
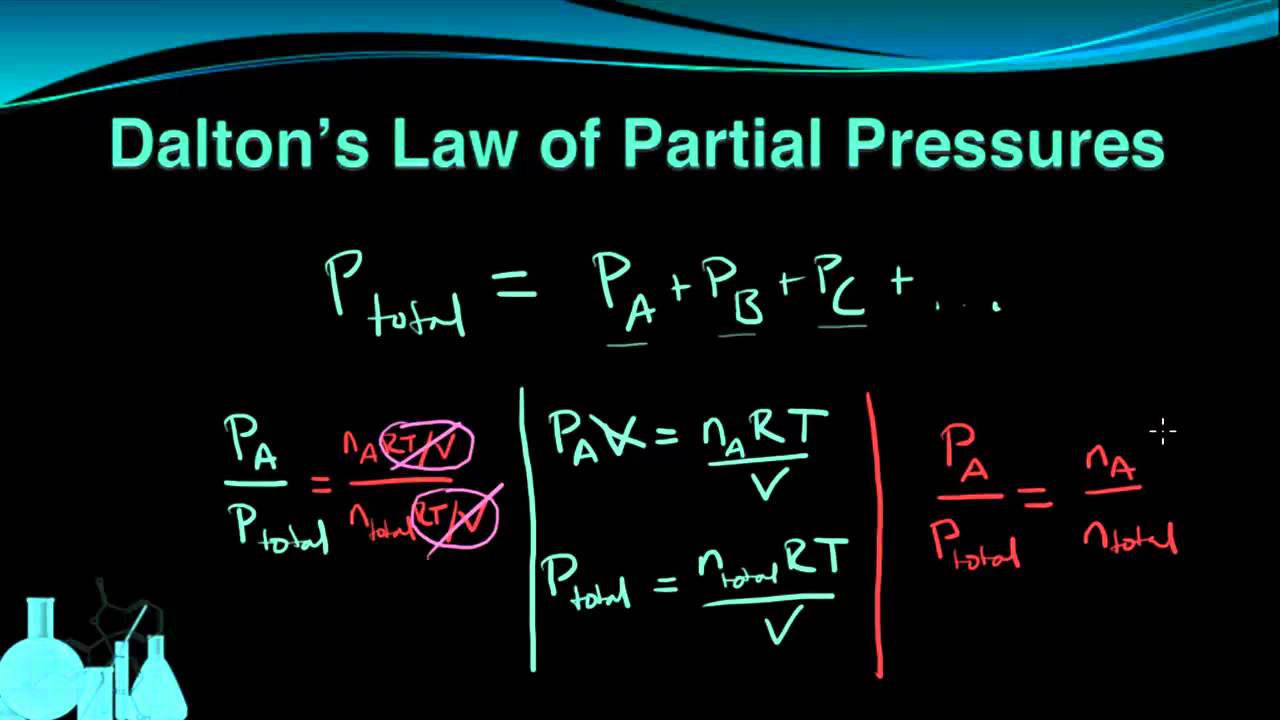
Chemistry 7 6 Dalton S Law Of Partial Pressures Dalton S Law Chemistry Dalton

Dalton S Law Of Partial Pressures Explained Dalton S Law Medical Anatomy Respiratory Therapy

15 12 6 Dalton S Law Of Partial Pressure In Mixtures Of Gases Each Component Gas Behaves Independently Of The Other S In 2022 Ideal Gas Law Molecular Physics Formulas
Comments
Post a Comment